Precision medical forecasting is emerging as a powerful frontier in medicine, offering personalized timelines for disease risk. It promises to predict when someone will develop major aging-related diseases. Examples include cancer, cardiovascular disease, and neurodegenerative diseases.
AI integrates biomarkers, body-wide aging clocks, organ clocks, and retinal scans. It also ingests electronic medical records and wearable sensor data. Therefore, clinicians could move from reactive care to proactive prevention. However, these advances rest on solid science and careful validation.
Immunosenescence and inflammaging shape risk over decades, so forecasting must respect these biological realities. Prospective clinical trials will confirm benefit and guide ethical deployment. As a result, precision medical forecasting offers hope for delaying or preventing disease through lifestyle changes and targeted therapies.
Optimistic yet cautious, the field could redefine primary prevention for aging-related disease while demanding rigorous testing and safeguards. Clinicians, patients, and regulators must collaborate to realize its promise responsibly. The next decade will be decisive.
Precision medical forecasting: aging and biomarkers
The three major aging-related diseases share long incubation phases and growing prevalence. They also present distinct prevention challenges. Bullet points help clarify key features.
- Cancer
- Often emerges after decades of accumulated DNA damage and clonal expansion.
- Therefore, early molecular signals matter for timing interventions.
- Cardiovascular disease
- Builds slowly through atherosclerosis, hypertension, and metabolic stress.
- As a result, risk prediction years ahead can change therapy and lifestyle choices.
- Neurodegenerative diseases
- Include Alzheimer’s and Parkinson’s.
- They show very long preclinical phases, so early biomarkers are essential.
Precision medical forecasting and immune aging
Aging alters immune function through immunosenescence and chronic inflammation. For example, thymic involution and T cell shifts weaken immune responses, and inflammaging raises systemic cytokines. These processes increase vulnerability to cancer, heart disease, and neurodegeneration. For more on mechanisms, see a review on immunosenescence: review on immunosenescence and additional insights.
Key molecular tools that power forecasting include:
- Biomarkers such as protein measures and blood tests. For Alzheimer’s, p-tau217 shows promise: study on p-tau217 and blood biomarkers.
- Body-wide aging clocks that aggregate multiomic signals across tissues.
- Organ clocks that track specific organ risk trajectories, like brain organ clocks for dementia.
Finally, AI links these signals to electronic medical records and sensors, which aids model training and deployment. For examples of AI in digital health and clinical workflows, see AI in digital health and Tower Plus AI.
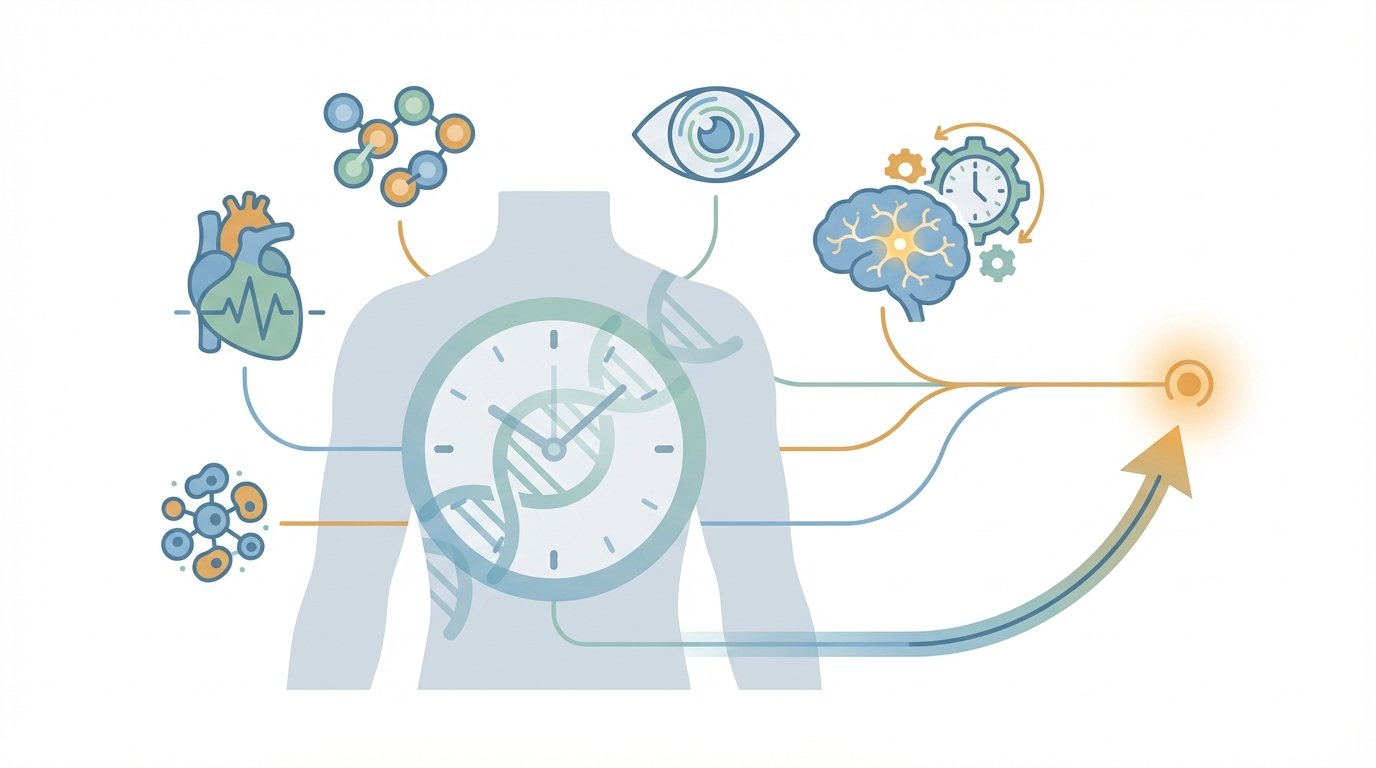
How artificial intelligence enables precision medical forecasting
Artificial intelligence brings many data streams together to forecast disease timing. It ingests electronic medical records, including structured fields and unstructured clinician notes. It also reads lab results, imaging scans, genetic data, wearable sensors, and environmental exposures. As a result, AI algorithms can detect subtle patterns that no single test shows.
Key data sources include:
- Electronic medical records: demographics, diagnoses, medications, and free text clinical notes.
- Lab results and protein biomarkers: serial measures reveal trends over years.
- Imaging and retinal scans: eye images carry vascular and neural clues linked to heart and brain risk. See Poplin et al. and a scoping review: https://pubmed.ncbi.nlm.nih.gov/39563905/.
- Genetic data and polygenic risk: provide inherited susceptibility that modifies trajectories.
- Wearable sensors and environmental data: continuous activity, sleep, pollution, and context refine risk scores.
AI algorithms fuse these modalities with multimodal models and time-aware methods. Therefore, models map individual trajectories rather than static risk. This mapping creates a temporal arc, or ‘when factor’, which projects when disease is most likely to appear. For example, retinal-based models predict cardiovascular events and neurodegenerative risk years before symptoms appear. See retinal imaging review: https://pubmed.ncbi.nlm.nih.gov/33090722/ and multimodal Alzheimer’s work: https://link.springer.com/article/10.1186/s13195-024-01668-5.
Because the temporal arc guides preventive choices, clinicians can time interventions and monitoring. However, prospective clinical trials must test whether acting on forecasts improves outcomes.
Table: Traditional Risk Scoring versus Precision Medical Forecasting
| Attribute | Polygenic risk scoring (traditional) | Precision medical forecasting |
|---|---|---|
| Data inputs | Genetic variants; single-timepoint | Multiomic biomarkers; labs; imaging; retinal scans; wearables; EHR time series |
| Model type | Statistical risk estimate; static | Multimodal AI models; time-aware forecasting |
| Temporal prediction | Risk probability only | Explicit timing; temporal arc ‘when factor’ |
| Accuracy and granularity | Population-level stratification; limited individual precision | Higher individual precision; organ-specific clocks; dynamic updates |
| Guidance for interventions | Broad recommendations; preventive screening | Personalized timing for lifestyle changes; targeted meds like GLP-1; monitoring plans |
| Typical outputs | Percentile risk; relative risk | Projected years-to-event; organ clock scores; actionable timelines |
| Validation | Established in cohorts; slower translation | Early-stage; needs prospective clinical trials |
| Practical impact | Low immediate behavior change | Enables timely lifestyle change and treatment selection |
Conclusion
Precision medical forecasting promises a step change in primary prevention for aging-related disease. It ties biomarkers, body-wide aging clocks, organ clocks, retinal scans, and wearable sensors into a single forecast. Therefore clinicians can move beyond risk percentages to a projected temporal arc. As a result, patients receive actionable guidance about when to intensify lifestyle change or start targeted therapies.
However this optimism must remain measured. Prospective clinical trials will determine whether forecasts change outcomes. Yet early evidence and mechanistic biology support cautious hope. Because immunosenescence and inflammaging drive long disease pathways, timing matters. Consequently, forecasts that predict years-to-event can shift prevention from guesswork to strategy.
EMP0 is a pioneering company that helps organizations build and deploy next-generation health forecasting and growth systems. EMP0 offers ready-made tools and AI-powered growth systems that speed productization. Importantly EMP0 supports secure deployment under client infrastructure to protect data and meet compliance needs. To learn more, visit EMP0 and explore the blog at EMP0 Blog. For automation integrations see EMP0 Automation Integrations.
In short precision medical forecasting could reshape prevention for cancer, cardiovascular disease, and neurodegeneration. With careful validation and ethical deployment, it can deliver earlier interventions and better outcomes.
Frequently Asked Questions (FAQs)
What is precision medical forecasting?
Precision medical forecasting uses biomarkers, AI, and clocks to predict when disease may appear. It projects a temporal arc for individual risk.
How does precision medical forecasting benefit patients?
It shifts care from reactive to proactive, allowing timed prevention and monitoring. Therefore, clinicians can personalize lifestyle and medication plans.
What technologies power precision medical forecasting?
Artificial intelligence analyzes electronic medical records, imaging, retinal scans, genetic data, wearable sensors, and protein biomarkers. Body-wide aging clocks and organ clocks add organ-specific timing.
Can lifestyle changes or medicines reduce forecasted risk?
Yes, lifestyle measures such as an anti-inflammatory diet, regular exercise, and sleep lower risk. Medications like GLP-1s may help, but clinicians should guide use.
Are precision forecasts clinically validated?
Early studies show promise, however prospective clinical trials are required. As a result, broad clinical adoption depends on trial confirmation.

